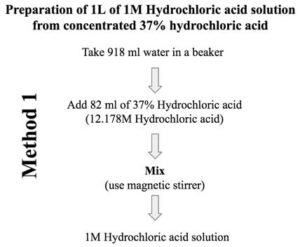
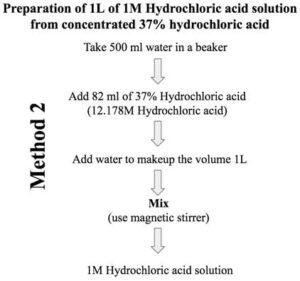
Discussion | 31 5. How many grams are needed to make 2.5L of 1.5M HCl solution? CI 350 レ. 6. How many grams are needed to make 500...

How to Prepare 1M HCl Solution | Preparation of 0.1M HCl Solution | Hydrochloric acid 0.1 M Solution - YouTube

A 37%(w/w) solution of Hydrochloric acid has a density of 1 18 g/mol What volume of this solution should - Chemistry - Some Basic Concepts of Chemistry - 13482901 | Meritnation.com
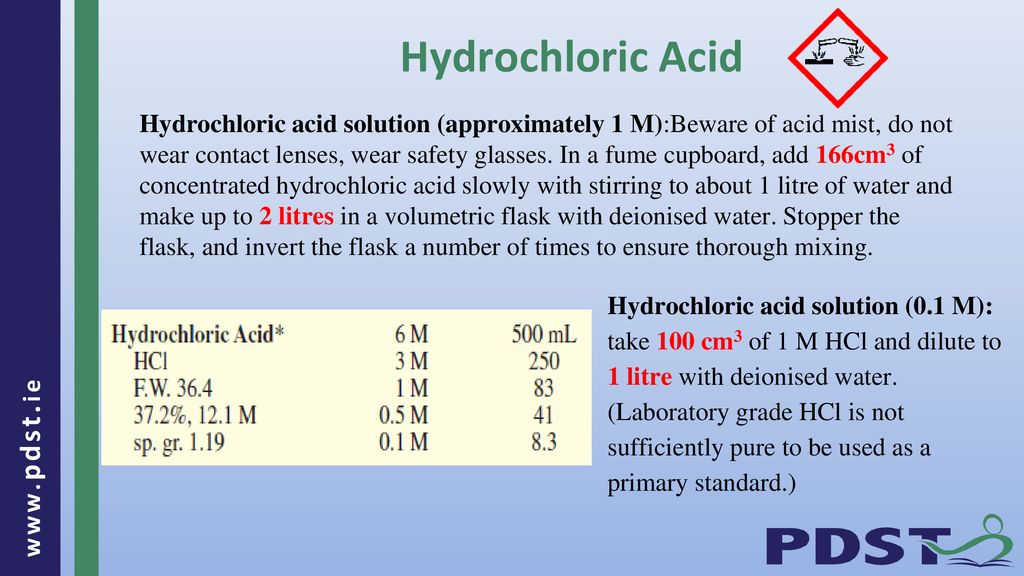
Hydrochloric Acid Hydrochloric acid solution (approximately 1 M):Beware of acid mist, do not wear contact lenses, wear safety glasses. In a fume cupboard, - ppt download

1. Which Vhich will basic buffer? 100 mL of 0.1 M HCl + 100 ml of 0 1 (a) 100 mL of o M NaOH (b) 50 mL of 0.1 M M

How to Prepare 1 molar HCl from 37% of HCl having density 1.18 g/cm3. | Umair Khan Academy - YouTube
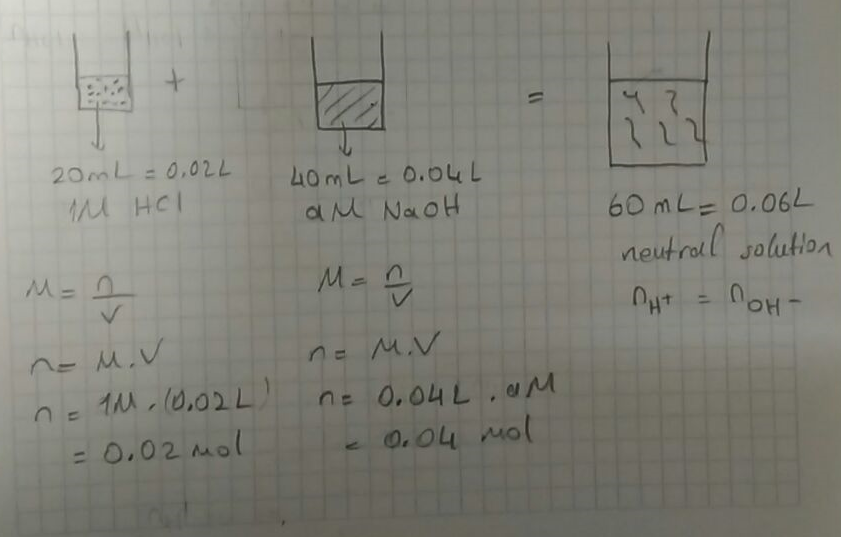
If 20 mL of 1.0 M HCl is used completely to neutralize 40 mL of an NaOH solution, what is the molarity of the NaOH solution? | Socratic
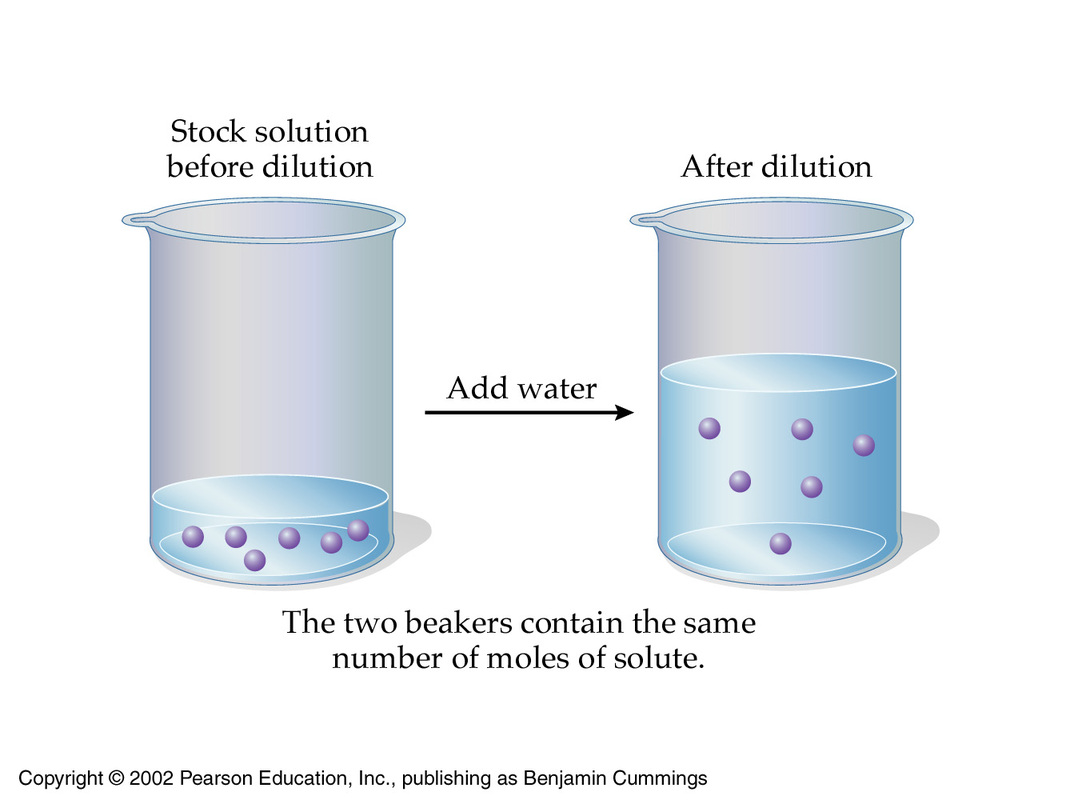
How many milliliters of 12.0 M HCl(aq) must be diluted with water to make exactly 500. mL of 3.00 M hydrochloric acid? | Socratic

SOLVED: Solution Preparation and Standardization Name: Hydriodic Acid Calculations Calculate the volume of concentrated (12 M) HCI required to make 100.0 mL of a 1M solution: CcVr = CaVd (12 M)(Vr) = (
1 M NH4OH and 1 M HCl are mixed to make total volume of 300 mL. If pH of the mixture is 9.26 and pKa (NH4^+) = 9.26 - Sarthaks eConnect | Largest Online Education Community